Abstract
Objective: To assess the efficacy of immunotherapy based on autologous dendritic cells-cytokine induced killer cells (DC-CIK) and allogeneic natural killer cells (NK) in treating low-risk and intermediate-risk acute myeloid leukemia (AML). The study was conducted over a period of 11 years (April 1, 2006 to April 1, 2017) across the Beijing Ludaopei hospital systems.
Methods: DC-CIK cells were derived by culturing patients' peripheral blood mononuclear cells (PBMCs) in vitro with cytokines for 8-12 days. To generate NK cells, allogenic PBMCs were cultured for 3 to 6 days. After patients completed intensified chemotherapy consisting of 4 cycles of high-dose Ara-C within 6 months, DC-CIK or NK cells were infused and given once every 3 months for 2-4 cycles along with chemotherapy of Fludarabine, Cyclophosphamide and Ara-C given few days prior to each cycle of immunotherapy.
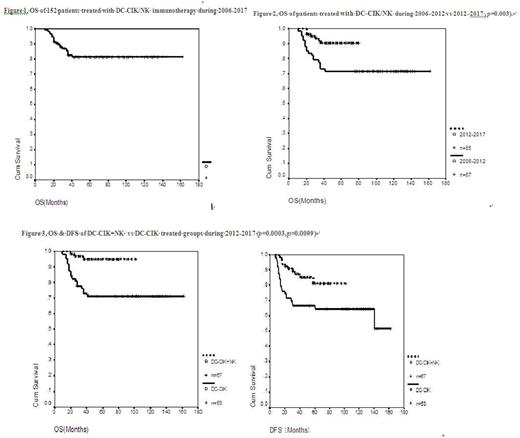
Results: From April 1, 2006 to April 1, 2017, a total 152 patients in the low and intermediate risk groups of AML (except APL) underwent combined immunotherapy and chemotherapy in our center. Overall survival (OS) rate was 82% and disease-free survival (DFS) rate was 67% for the cohort. Beginning in June 2012, the low and intermediate risk groups were further stratified into very-good risk, good risk, and intermediate risk groups according to the specific AML related gene mutations. The OS rate of the 2012-2017 group was significantly better than that of the 2006-2011 group (91.7% vs. 71.6% p= 0.003). For patients treated prior to 2012, there were no significant differences between the low and intermediate risk group in OS (72.2% vs 71.4%) and DFS (72.2% vs 61.2%). From 2012 to 2017, the OS rates were 94.4%, 86.3%, and 93.3%, and the DFS rates were 83.3%, 81.8%, and 62.2%, (p=0.15) for the very-good risk, good-risk & intermediate risk groups, respectively, again suggesting no statistical differences among the groups. Side effects were mild with some fever, chills and fatigue.
Patients who were treated with 2-4 cycles of immunotherapy were further divided into the DC-CIK alone group and the DC-CIK alternating with NK group. Importantly, the OS and DFS rates of 67 patients in the DC-CIK alternating with NK group were significantly better than those of 53 patients in the DC-CIK alone group (OS 95.5% vs 71.4%, p=0.0003), (DFS 85% vs 63.5%, p =0.0099). .
Twenty-nine of 48 relapsed patients from both groups underwent allo-hematopoietic stem cell transplantation (allo-HSCT). The long-term OS after HSCT was 65.5%, closed to the OS of AML patients who transplanted in their first complete remission (CR) in our center. OS of 12 patients transplanted after 2012 was significantly better than that of 17 patients transplanted before 2012 (88.2% vs. 33.3%, p = 0.015). This because that after 2012, all patients have been checked minimal residual disease (MRD) by flow cytometry. Once patient was found to have relapse with MRD,immediately pursued HSCT while some patients transplanted prior to 2012 had morphological relapse.
Conclusion: When combined with chemotherapy, DC-CIK/NK cells-based immunotherapy significantly improves the long-term OS and DFS rates of good and intermediate risk groups of AML. Auto DC-CIK alternating with allo NK is superior to auto DC-CIK alone. Relapsed patients could be safely and effectively treated with HSCT. If relapse patients with only positive MRD, then the outcome of HSCT will be comparable to those who received the HSCT during their first CR.
Figure 1. OS of 152 patients treated with DC-CIK/NK immunotherapy from 2006 to 2017.
Figure 2, OS of 67 patients treated from 2006 to 2012 vs 85 patients from 2012 to 2017 (71.6% vs 91.7%, p= 0.003).
Figure 3, OS & DFS of DC-CIK+NK vs DC-CIK treated groups from 2012 to 2017 (p= 0.0003, p= 0.0099)
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal